Use for electroplating power supplies and rectifiers
Electroplating, also known as electrodeposition, is the process of depositing a layer of metal on the surface of an object by passing an electric current through a solution containing dissolved metal ions. This process has a variety of applications, ranging from decorative purposes to enhancing specific properties of objects. Here's a look at some of the more prominent applications:
Diamond Tool production -🔗Diamond tools utilize the exceptional hardness of diamond, the hardest known natural material, to achieve high performance cutting, grinding, drilling and polishing. These tools are used in a wide range of applications due to their durability, precision and effectiveness in working with hard or abrasive materials. They are usually electroplated or electroformed. |
 |
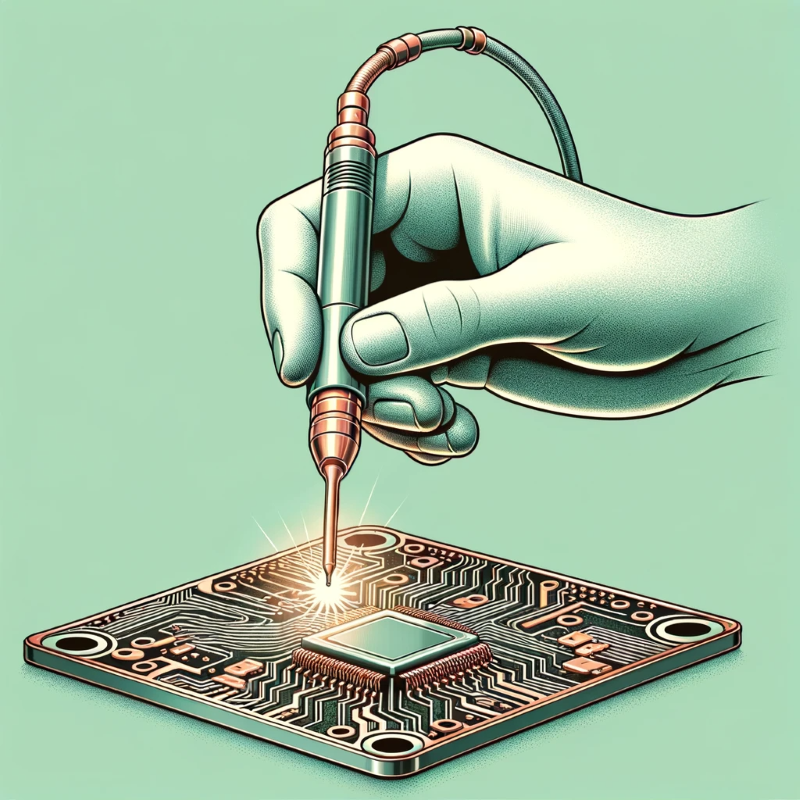
Electrical conductivity - 🔗Certain applications require improved electrical conductivity. By electroplating with metals known for their high conductivity, such as copper or silver, the efficiency of electrical connections can be improved. This is critical in the electronics and telecommunications industries. |
 |
Printed circuit board (PCB) manufacturing - 🔗Electroplating is critical in the electronics industry, especially in the manufacture of printed circuit boards. Copper plating is used to create conductive paths on these boards that connect various electronic components. |
 |
Decorative purposes - 🔗Electroplating is widely used to enhance the appearance of objects by giving them a shiny, reflective surface. For example, jewelry, watches and other fashion accessories are often plated with precious metals such as gold or silver to enhance their aesthetic appeal. |
 |
Wear resistance - 🔗Plating can be used to coat surfaces with a layer of harder metal, increasing their resistance to wear. This is especially important for parts that experience regular friction, such as gears, bearings or engine components. Chromium is a popular choice for such applications due to its high hardness. |
 |
Corrosion protection - 🔗Some metals, such as steel, are susceptible to corrosion when exposed to environmental conditions such as moisture or certain chemicals. By plating them with non-corrosive metals such as nickel, zinc or gold, their life can be significantly extended. This is particularly beneficial in industries such as automotive, where parts are exposed to varying weather conditions. |
 |
Reduced friction - 🔗Some electroplated coatings, such as those using molybdenum or tungsten disulfide, can significantly reduce friction between mechanical parts. This not only extends the life of these parts, but also improves their efficiency. |
 |
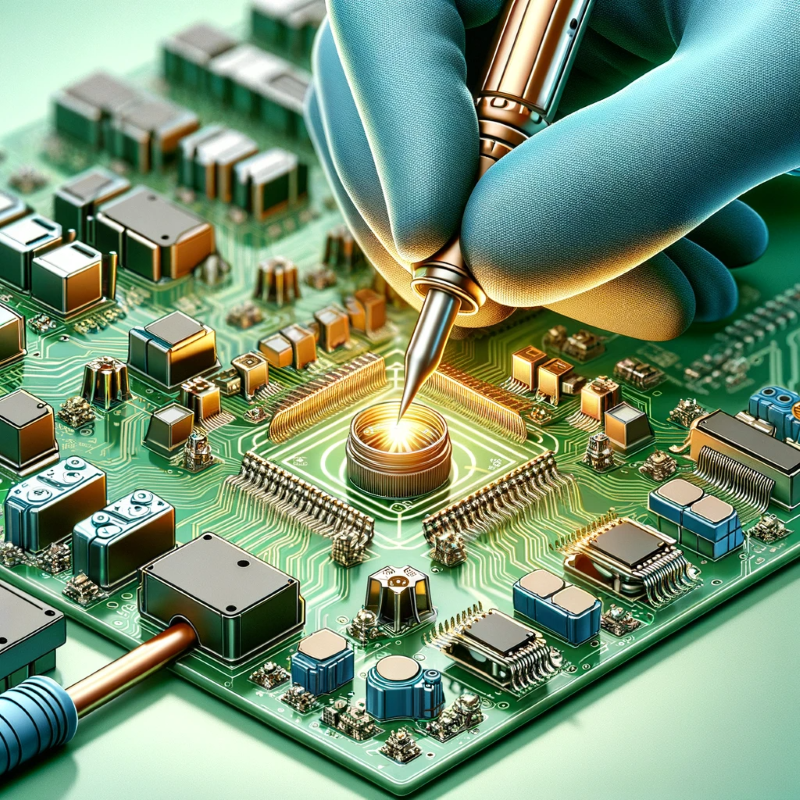
Solderability - 🔗In electronics manufacturing, components often need to be soldered to circuit boards. Electroplating with tin or lead (although lead is being phased out due to environmental concerns) can make the soldering process more effective by providing a stronger bond between components. |
 |
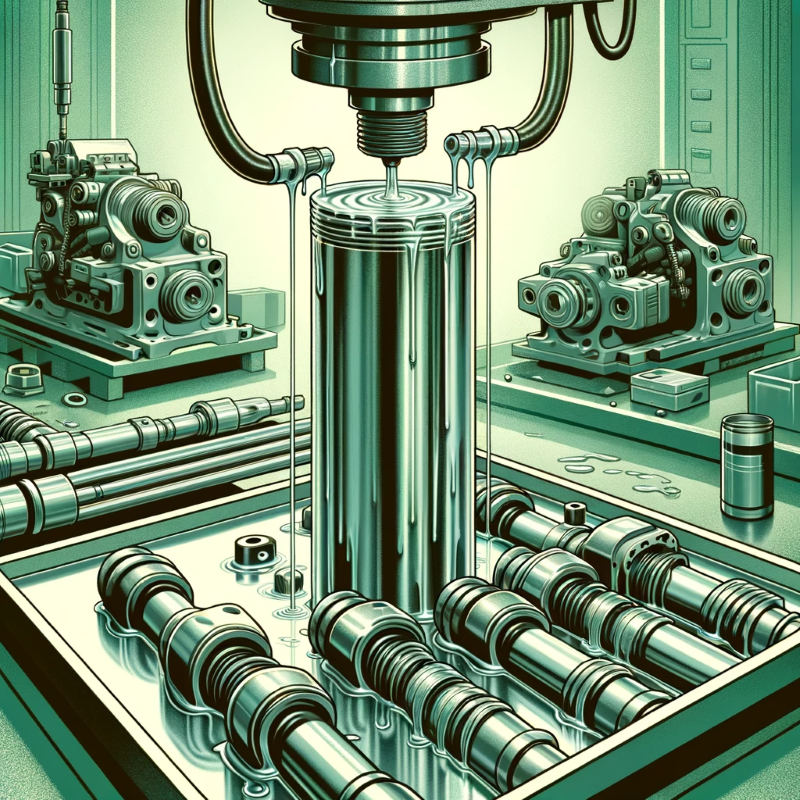
Hard Chrome Plating - 🔗In addition to its decorative uses, chrome can be plated in thicker layers to harden surfaces. This "hard chrome plating" is used in hydraulic cylinders, shafts, and other components to increase durability and reduce wear. |
 |
 x 0,4A / 0-12V
x 0,4A / 0-12V  x 25A / 0-10V
x 25A / 0-10V  Multi-channel power supply
Multi-channel power supply  German
German 